In linear pharmacokinetics (PK), when we increase the dose (D) of the drug, its level in our body or systemic exposure (AUC) increases proportionally (red curve on left graph). What is our inference?
This implies that both oral bioavailability (F) and clearance (CL) are constant such that dose (D) and systemic exposure (AUC) are proportional to one another! Here, we can easily adjust the dose to reach a target exposure! Wow nice!
Furthermore, the rate of elimination is proportional to the plasma drug concentration (C) since the proportionality constant is the constant clearance (CL) (red curve on right graph). Makes sense too!
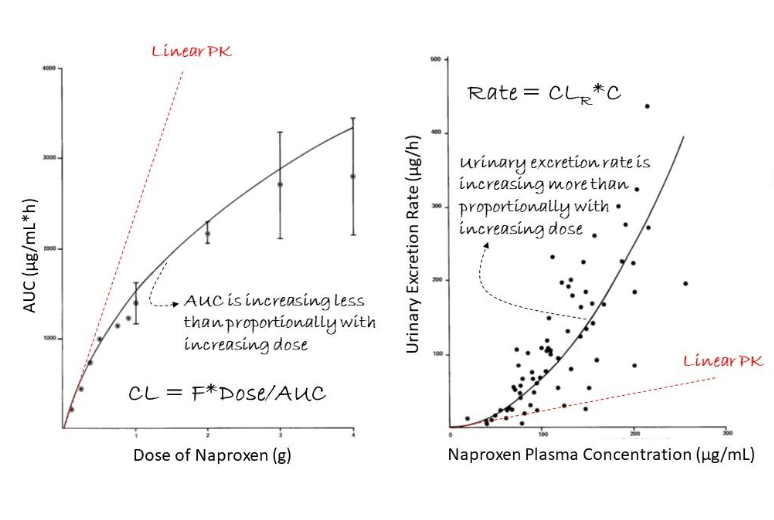
So what is happening in the case of naproxen, an anti-inflammatory drug?
First, its systemic exposure increases less than proportionally than the increase in dose (D) (black curve on left graph).
Second, its excretion rate in urine increases more than proportionally than the increase in plasma concentration (C) (black curve on right graph).
How should we interpret the data?
This is a case of nonlinear PK of naproxen where it shows dose-dependency. For example, the renal clearance (CLR) of naproxen increases with increasing dose (i.e. CLR is dose-dependent).
But why does the CLR of naproxen change with dose?
For an acidic drug like naproxen (see structure), it is extensively bound to plasma protein (albumin). At the clinical doses, the plasma concentration of naproxen is sufficient to saturate the plasma protein binding. With increasing plasma concentration, its unbound fraction in plasma (fu) increases. This increase in fu in turn leads to an increase in both (1) glomerular filtration clearance (fu*GFR) and (2) active secretory clearance by transporter in the kidney. Wow what a nice explanation!
I hope we appreciate the differences between linear and nonlinear PK using this example.
Read also:
- Relation Between Renal clearance and Urine pH
- Food-Drug Interactions on Drug Safety & Efficacy
- Dietary Reference Intakes for Vitamin C (Ascorbic Acid)
Resource Person: Eric Chan, PhD

